MAGNITUDE Study Design2
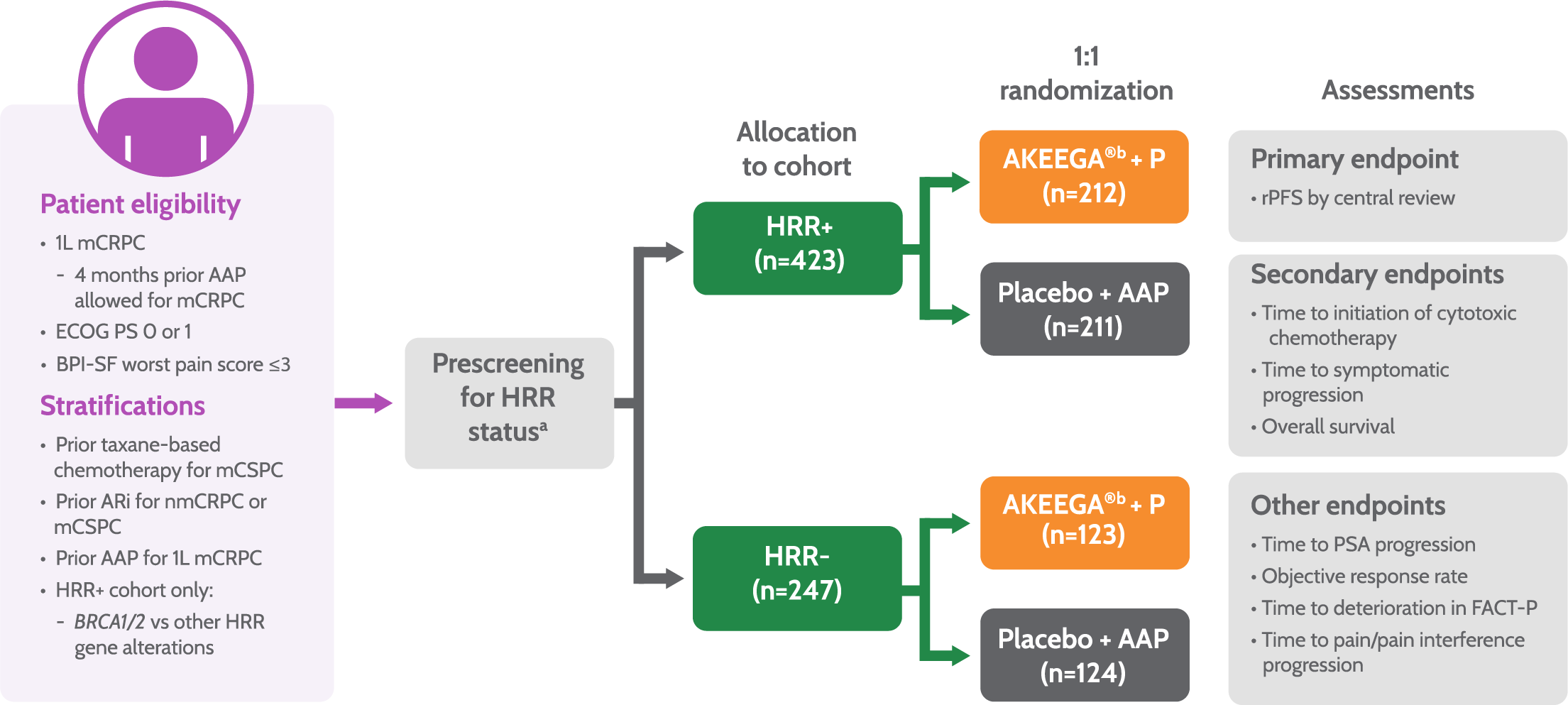
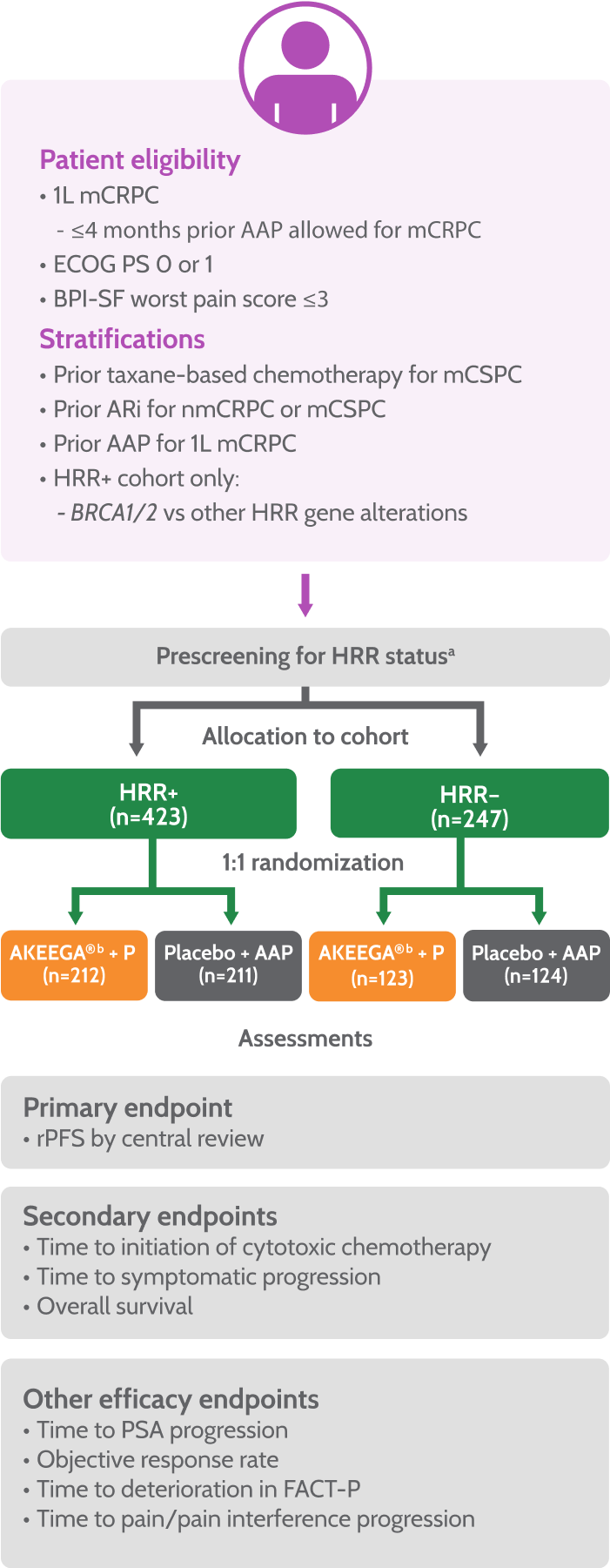
53% (225/425) of patients enrolled in the HRR+ cohort had BRCA gene mutations.1
Study start: February 2019; clinical data cutoff for Interim Analysis 1: October 8, 2021, Interim Analysis 2: June 17, 2022, Final Analysis: May 15, 2023.6
aPatients were prospectively tested by plasma, tissue, and/or saliva/whole blood. Patients negative by plasma only were required to test by tissue to confirm HRR BM status. Tissue and plasma assays used included FoundationOne tissue test (FoundationOne®CDx), Resolution Bioscience liquid test (ctDNA), AmoyDx® blood and tissue assays, Invitae germline testing (blood/ saliva), or local lab BM test results demonstrating a pathogenic germline or somatic alteration outlined in the study protocol.
bPatients in these cohorts received niraparib 200 mg once daily with AA 1,000 mg once daily plus prednisone 5 mg twice daily.
Patients enrolled in the MAGNITUDE trial were prospectively tested for HRR status1,3
Patients were tested using FoundationOne®CDx tissue assay or other clinical trial assays
Enrollment in non-HRR-altered cohort was stopped when predetermined criteria of the futility analysis were met






Over half of patients enrolled in MAGNITUDE Cohort 1 had BRCA1/2 mutations1,4
Baseline characteristics in BRCAm patients | AKEEGA® + P (n=113) | Placebo + AAP (n=112) |
| Age, median (range), y | 67 (45-100) | 68 (43-88) |
| ECOG PS 0 / 1, n (%) | 69 (61.1) / 44 (38.9) | 80 (71.4) / 32 (28.6) |
| Bone metastases, n (%) | 99 (87.6) | 93 (83.0) |
Visceral metastases, n (%)
| 26(23.0)
| 22 (19.6)
|
| PSA at study entry (μg/L), median (range) | 18.7 (0.1-2225.8) | 14.1 (0.1-4400.0) |
| Prior taxane-based chemotherapy for nmCRPC/mCSPC, n (%) | 26 (23.0) | 29 (25.9) |
| Prior AR-targeted therapy for nmCRPC/mCSPC, n (%) | 6 (5.3) | 5 (4.5) |
| Prior AAP therapy for 1L mCRPC,a n (%) | 30 (26.5) | 29 (25.9) |
AKEEGA® + P
30 (26.5)
Placebo + AAP
29 (25.9)
aPatients could have received up to 4 months of AAP in the mCRPC setting before study entry.
aPatients could have received up to 4 months of AAP in the mCRPC setting before study entry.
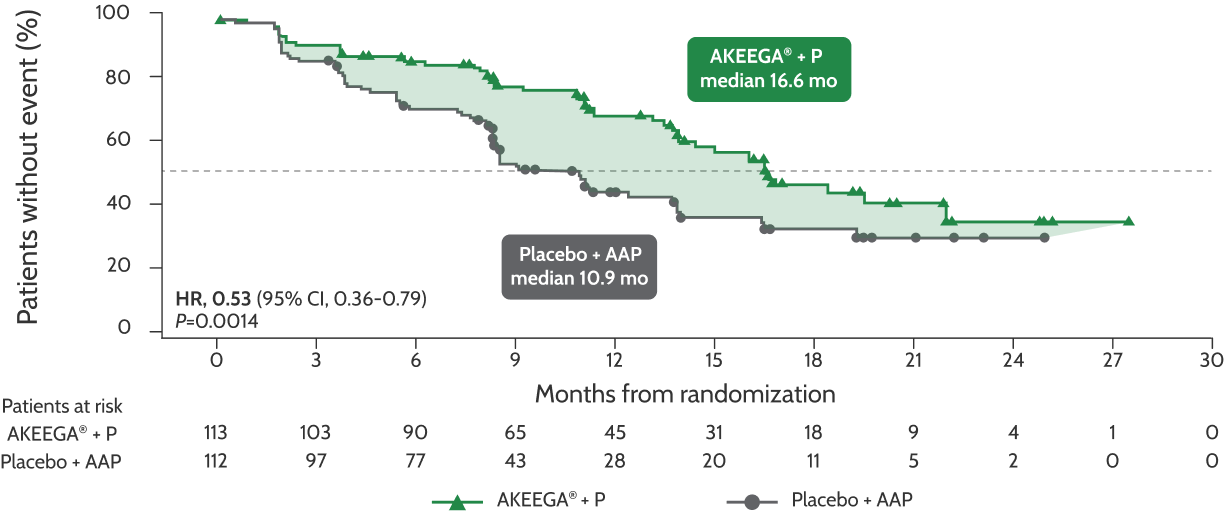
Primary Analysis‡: Primary Endpoint
AKEEGA®* significantly reduced risk of disease progression or death in BRCAm mCRPC patients1
47%
reduced risk of disease progression or death
HR, 0.53 (95% CI, 0.36-0.79); P=0.0014




The median in the AKEEGA®* arm was 30.4 months (95% CI, 27.6-NE) and 28.6 months (95% CI, 23.8-33.0) in the placebo + AAP arm, with an OS hazard ratio of 0.79 (95% CI, 0.55-1.12)
Statistical significance has not been established for these endpoints. They should be viewed in the context of patient management, overall physical condition, and clinical course of the patient.





AKEEGA® has a manageable safety profile1
Use biomarker testing to determine your patient’s mutation status1
*AKEEGA® is indicated with 10 mg prednisone daily.1
†Patients were allowed up to 4 months of prior AAP in the mCRPC setting.1
‡The median duration of follow-up in the BRCA1/2 subgroup at the primary analysis was 16.7 months.5
§The median follow-up in the BRCA1/2 subgroup at interim analysis 2 was 24.8 months (range 0.5-36.8 months).4
‖The OS HR and median OS were determined in an exploratory analysis.1
1L = first-line; AAP = abiraterone acetate (AA) + prednisone (P); ARi = androgen receptor (AR) inhibitor; BICR = blinded independent central radiology; BM = biomarker; BPI-SF = Brief Pain Inventory-Short Form; BRCAm = BRCA gene-mutated; CI = confidence interval; ctDNA = circulating tumor DNA; ECOG PS = Eastern Cooperative Oncology Group Performance Status; FACT-P = Functional Assessment Cancer Therapy-Prostate; HR = hazard ratio; HRR = homologous recombination repair; KM = Kaplan-Meier; mCRPC = metastatic castration-resistant prostate cancer; mCSPC = metastatic castration-sensitive prostate cancer; NE = not estimable; nmCRPC = non-metastatic castration-resistant prostate cancer; OS = overall survival; PSA = prostate-specific antigen; rPFS = radiographic progression-free survival.
References:
- AKEEGA® [Prescribing Information]. Horsham, PA: Janssen Biotech, Inc.
- Efstathiou E, Smith MR, Sandhu S, et al. Niraparib with abiraterone acetate and prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of MAGNITUDE. Poster presented at: the American Society of Clinical Oncology Genitourinary (ASCO-GU) Cancers Symposium; February 16-18, 2023; San Francisco, CA, USA.
- Chi KN, Rathkopf D, Smith MR, et al. Niraparib and abiraterone acetate for metastatic castration-resistant prostate cancer. J Clin Oncol. 2023;20;41(18):3339-3351. doi:10.1200/JCO.22.01649
- Chi KN, Sandhu S, Smith MR, et al. Niraparib plus abiraterone acetate with prednisone in patients with metastatic castration-resistant prostate cancer and homologous recombination repair gene alterations: second interim analysis of the randomized phase III MAGNITUDE trial. Ann Oncol. 2023;S0923-7534(23)00757-3. doi:10.1016/j.annonc.2023.06.009
- Chi KN, Rathkopf DE, Smith MR, et al. Phase 3 MAGNITUDE study: First results of niraparib (NIRA) with abiraterone acetate and prednisone (AAP) as first-line therapy in patients (pts) with metastatic castration resistant prostate cancer (mCRPC) with and without homologous recombination repair (HRR) gene alterations. Presented at: the American Society of Clinical Oncology Genitourinary (ASCOGU) Cancers Symposium; February 17-19, 2022; San Francisco, CA, USA.
- Chi K, Castro E, Attard G, et al. Niraparib (NIRA) with abiraterone acetate plus prednisone (AAP) as first-line (1L) therapy in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) and homologous recombination repair (HRR) gene alterations: three-year update and final analysis (FA) of MAGNITUDE. Oral presentation presented at: European Society of Medical Oncology (ESMO) Congress; October 20-24, 2023; Madrid, Spain.




